Which Best Describes Elevctrolytes and Nonelectrolytes in Solutions
Solutions that do not conduct electricity are called non -electrolyte solutions. How do you determine if a base is strong or weak.
Strong electrolytes partially ionize in solution.
. Yet covalently bound materials where. Which best describes electrolytic and nonelectrolyte solutions. Which term best describes the thornscrub ecosystem.
After equilibrium is established the container is opened to allow the NH3 g and HCl g to escape. As a consequence electrolyte solutions conduct electricity readily. We review their content and use your feedback to.
The final formula to convert 50 Fahrenheit to Celsius is. All electrolytes are ionic substances. A strong electrolyte generates a current of electrons in solution.
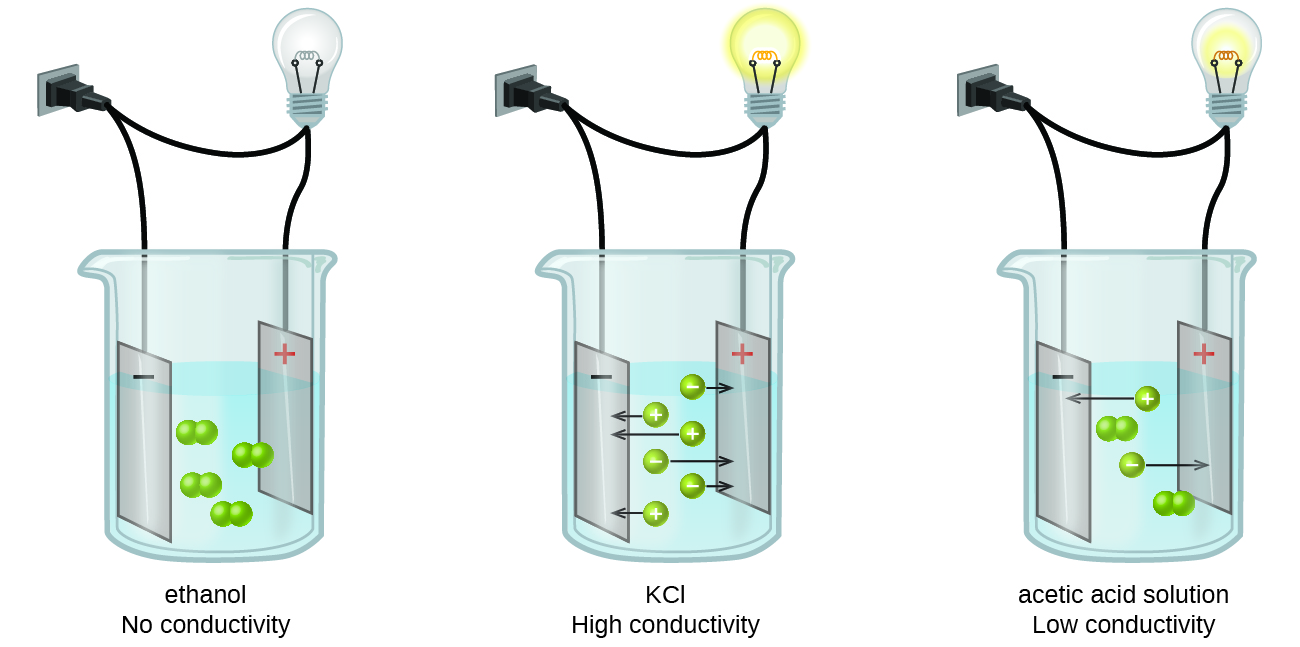
Hence no electrolyte solutions do not conduct electricity. Nonelectrolytes are chemical compounds that do not conduct electricity when dissolved in water. An electrolyte is a substance that produces an electrically conducting solution when dissolved in a polar solvent such as water.
Even insoluble ionic compounds such as CaCO 3 are electrolytes because they can conduct a current in the molten melted state. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Covalent electrolytes are typically.
1 blue 3 orange 2 yellow 4 red 27 Given the reaction. Electrolytes are salts or molecules that desociates into its ions in solution as a result they conduct electricity while Non electrolyte s dont desociates into ions in solution and as a result dont conduct electricity Sugar solution is non ele. Depends on the substance Covalent electrolytes can be strong or weak depending on the strength of the acid or base.
Experts are tested by Chegg as specialists in their subject area. Many molecular compounds such as sugar or ethanol are nonelectrolytes. Strong acids and bases are typically strong electrolytes.
Electrolytic solutions produce ions in solution while nonelectrolytes do not produce ions in solution. Report Table 1 Conductivities and Electrolyte Classifications for Solutions Examined in Part A. Which statement about electrolytes and nonelectrolytes is not true.
To convert Celsius to Fahrenheit simply multiply the Celsius value by 95 or 18 and then add 32 with it. Solutions of electrolytes contain ions that permit the passage of electricity. Some molecular substances are electrolytes.
Compounds that dissolve in water. Answer choices The solute particles are so firmly bonded that they do not break apart. Nonelectrolyte solutions do not therefore conduct electricity.
- 2804172 bre160 bre160 02042017 Chemistry. These ions can conduct electricity through the solution. A strong electrolyte is a substance that splits water into hydrogen and oxygen by electrolysis.
Solutions of nonelectrolytes such as ethanol do not contain dissolved ions and cannot conduct electricity. The main difference between electrolytes and nonelectrolytes is that electrolytes can get ionized when dissolved in water whereas. Dorling Kindersley Getty Images.
Common Ion Effect and Spectator Ions Watch on. Which of the following describes a solution containing an electrolyte. But the pure water or distilled water not includes in the type of electrolytes as they cannot form the ions in the solutions.
In solution nonelectrolytes do not dissociate from ions. A strong electrolyte is a substance that dissociates or ionizes completely when dissolved in water. Answer choices The solute particles are so firmly bonded that they do not break apart.
These solutions conduct electricity because they contain a solute that has dissociated into ions charged particles. The solute particles permit the passage of an electric current. It cannot contain any more solute particles.
It cannot contain any more solute particles. Weak electrolytes include weak acids weak bases and a variety of other compounds. Most soluble salts acids and bases are electrolytes.
Let us understand the relationship between Celsius and Fahrenheit. Solution identity and concentration conductivity μ Scm electrolyte classification Deionized water 26 nonelectrolyte Tap water 805 weak 004 M acetic acid CH3COOH 305 weak 004 M sucrose C12H12O11 16 nonelectrolyte 004 M hydrochloric acid. Nonelectrolytes do not dissociate into ions in solution.
Electrically such a solution is neutral. Some molecular substances are electrolytes. Electrolytes are also called as the polar compounds.
The dissolved electrolyte separates into cations and anions which disperse uniformly through the solvent. The conductivity of an electrolyte solution is related to the strength of. The ions of electrolytes have the ability to move in the electrolytic bath to do several changes in the solutions.
Its types are strong electrolytes and weak electrolytes. The solutes in these solutions dissolve as molecules neutral particles neither positively nor negatively charged. It is unstable and all the solute will precipitate if the solution is disturbed.
Is 50 degrees Celsius too hot. Who are the experts. If a solution prepared by dissolving 20 g of NaCl in 1 L of water.
Electrolytes are salts or molecules which in solution ionise completely. Weak electrolytes only partially break into ions in water. Compounds that dissolve by breaking into ions and conduct electricity in solution are known as electrolytes.
This is because they do not form ions when dissolved in water. Which of the following describes a solution containing an electrolyte. 1 Arrhenius acid and an electrolyte 2 Arrhenius acid and a nonelectrolyte 3 Arrhenius base and an electrolyte 4 Arrhenius base and a nonelectrolyte 26 According to Reference Table M what is the color of the indicator methyl orange in a solu-tion that has a pH of 2.
As electrolytes ionically bound substances act. It is unstable and all the solute will precipitate if the solution is disturbed. Which of the following concerning electrolytes and nonelectrolytes isare true.
9000 feet 8000 feet Temperate Forest 7000 feet 6000 feet Madrean Woodland sporadically. A strong electrolyte is any ionic substance. The solute particles permit the passage of an electric current.
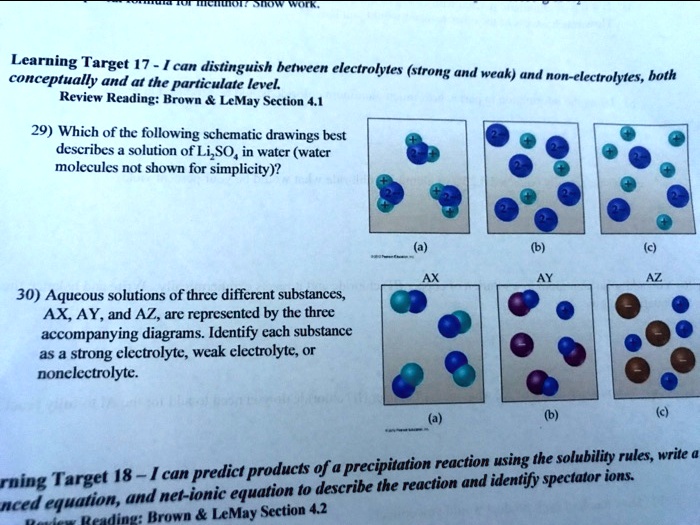
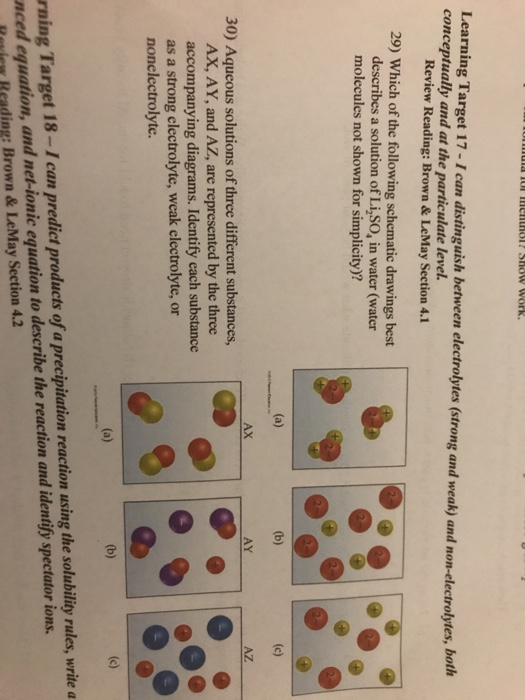
Solved Learning Target 17 Can Distinguish Between Electrolytes Strong And Weak And Conceptually And At The Particulate Level Non Electrolytes Hoth Review Reading Brown Lemay Section 4 1 29 Which Of The
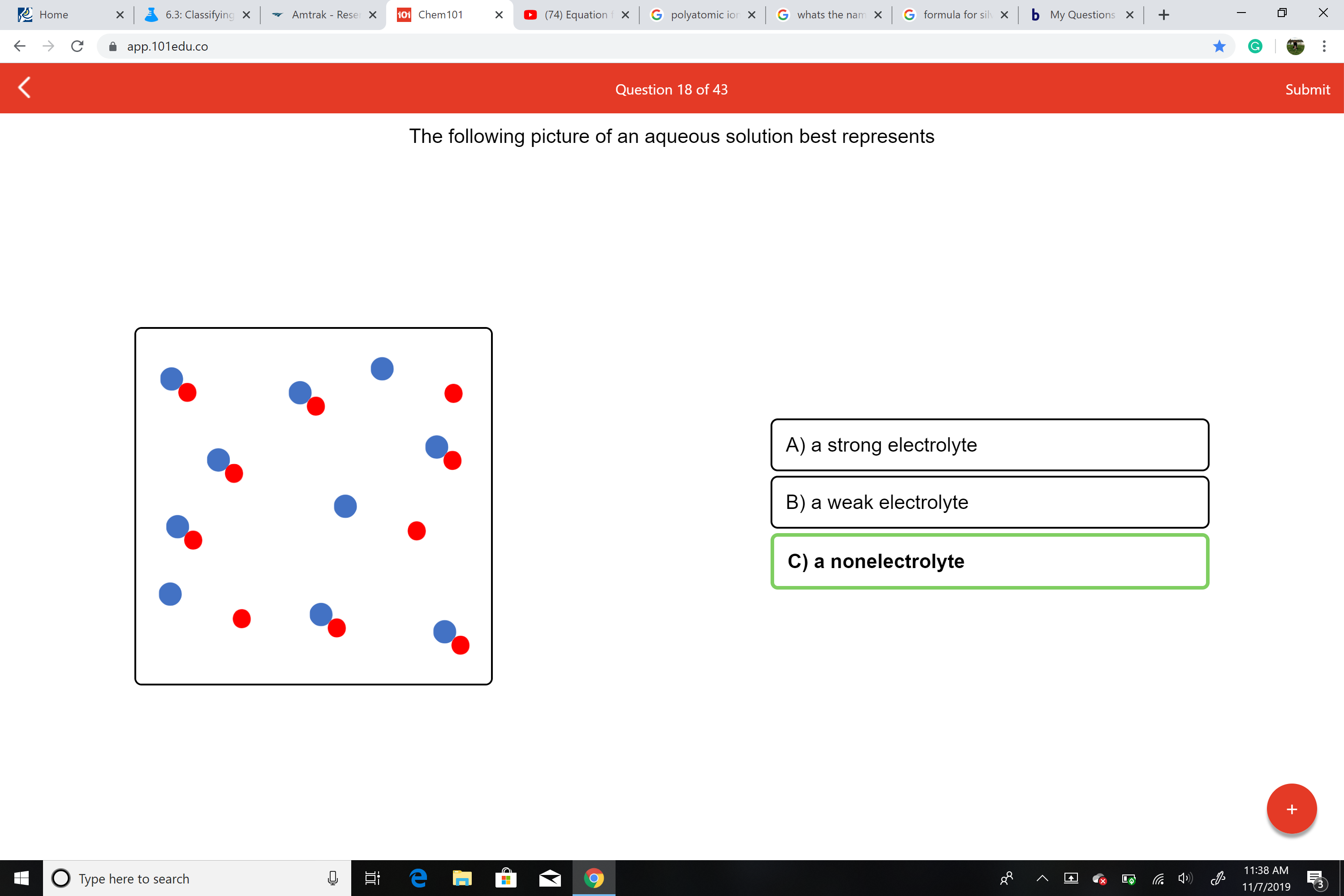
No comments for "Which Best Describes Elevctrolytes and Nonelectrolytes in Solutions"
Post a Comment